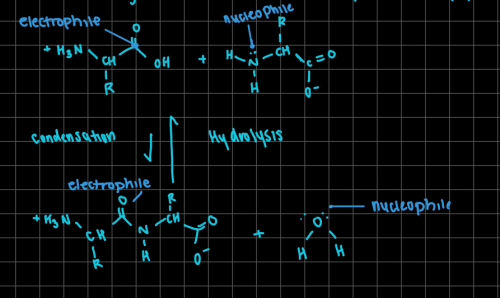
The condensation reaction between 2 amino acids to form a peptide bond or the hydrolysis reaction for a peptide
-- id the nucleophile and electrophile in both reactions
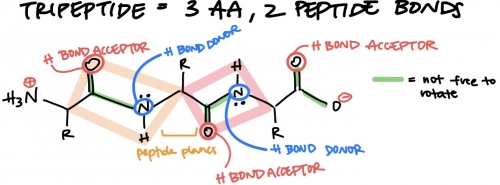
How many amino acids are in tripeptide? Draw it put w/ side chains labeled R.
--- id peptide plane, charged groups, and H bond donors/ acceptors. Which bonds in main chain can freely rotate and why cant the peptide bond freely rotate?
3 amino acids and 2 peptide bonds. peptide bond cant freely rotate bx carbonyl carbon and nitrogen are in a pi bond which would require signficicant amount of energy to rotate. it is possible, but extremely difficult to do. the main chains sigma bonds are able to freely rotate bc it doesnt require as much energy.
given a dG, state if a reaction is favorable. if reaction A has a dG of -10 kJ mol^-1, and reaction B has a dG of -5kJ mol^-1, which is faster?
reaction A is more favorable than B. however, that doesnt given any information in relation to time. therefore, comparing them is of no use in determining which reaction is faster. ----- dG is only dependent on the conditions of the reaction and the equilirbium constant: dG= dg^0 + RT ln Keq
under standard conditions, is the equation dG=dG^0 +RT ln Q the same as dG^0=-RT lnKeq? in other words, is the dG equation the general form of the free energy equation. but for all states?
if the reaction begins at unity in standard state conditions then RT lnQ =0. therefore, the first equation does simplify to dG=dG^0. so yes, dG serves as the general free energy equation taking into account changes in states
what limits the rate of a reaction?
----be able to id the limiting state on a free energy plot.
the reaction rate is limited by the stability of the transition state(s) thus the limiting state on a free energy plot has the highest free energy is the most unstable and the rate limiting transition state.
what is enthalpy? entropy? what is an exothermic reaction? endothermic?
enthalpy is the amount of heat that is absorbed or released. enthalpy is the change in allowable systems--- can be simply considered a measure of bond energy. enthalpy is the amount of heat or bond energy that is absorbed or released in a reaction. when dH is positive, heat is abosrbed and bond energy is gained and there is more energy for a certain bond to break (since breaking bonds always requires energy). when dH is negative, heat is released and bond energy is released, and when bond energy is released then bonds can be formed.
an endothermic reaction absorbs heat, and an exothermic reaction releases heat.
entropy dS is the change in allowable states of a system which are also known as degrees of freedom. a system that has a positive dS is known as favorable/disordered.
if a reaction is exothermic, it is always favorable?
no, an exothermic reaction can be unfavorable bc of dG of the reaction depends on both enthalphy and entropy. if the reaction is exothermic (enthalpy is negative) but the entropy is a larger negative then the dG of the reaction will be positive.
-------- equation: dG= dH - T dS
------ under what circumstances is an endothermic reactions exergonic?
an endothermic reaction can be favorable if the entropy of the system is large and positive. according to dG = dH - T dS, if enthalpy is positive due to it being an endothermic reaction, the reaction can still be exergoinc (dG is negative) if dS is large.
---- ice melting
why is cis-azobenzene a higher energy molecule than trans-azobenene?
the close proximity of the two benzenes on the cis molecule leads to a steric clash occuring, which causes angle strain on the double bond thereby making it less stable than an ideal double bond. the trans-azobenzene doesn't have the steric clash reaction, making it more stable relative to is cis counterpart. stability and energy levels are inversely related so the unstable cis molecule has more energy compared to its stabler trans counterpart.
--- the strain is manifest in the bond angels being distorted from ideality (their ideal values)
can bond breaking release energy to do work? how do molecules like peptides, anhydrides, phosphoanhydrides, mixed anhydrides, and thioesters release energy during hydrolysis?
no bond breaking never releases energy because it costs energy to break a bond. these molecules release energy during hydrolysis because they are actually swapping the bonds with water molecules/H+ molecules which is bond formation. when bonds are formed is when the energy is released, and since hydrolysis includes water molecules bonding, it therefore releases energy
------in reactions that release energy such as hydrolysis, the difference in energy released by new bonds created and the energy absorbed to break the previous bonds in great enough that the net bond swapping that occurs in the hydrolysis reaction makes the reaction exergonic
consider the dissociation reaction: HA <-> A^- + H^+. What does the equilibrium mean (what exactly is equal)? is the reaction still occurring at equilibrium?
equilibrium means the forwards and reverse rates of the reaction are equal to one another. the reaction is still occurring at equilibrium.
the reaction is still occurring, but there is no net velocity with respect to product formation or reaction formation. v(t) = k1 [reactant]-[product].
--- tug of war contest bw equally matched terms; the rope is twitching back and forth a little bit, so there is no net movement over time, but the teams are still pulling
what is the thermodynamic rule of thumb?
for every tenfold increase in products to reactants, there is a -5.7 k/mol increase in free energy. for every tenfold decrease in products to reactants, the is a -5.7 kj/mol decrease in free energy
the reacion A<--> B has a Keq of 1/1000. if you started the reaction at a Q of 1, using the rule of thumb, what would be the dG and dG^o of this reaction?
since the reaction starts at a Q of 1, dG and dG^0 are going to be equal (about 18 kj/mol). the dG^0 of this reaction is also positive, which means that its endogonic (reactant favored) at standard conditions.
------- dG = dG^0 + RT lnQ, plug in respective values. RTlnQ = 0. therefore: dG = dG^0. this makes sense bc dg^0 is defined as the energy change from a reaction at equal concentrations and standard conditions (Q1) to equilibrium. ---- the reaction defined as endergonic (nonspontaeous) is behaving spontaneously.
------------------- a reaction w/ unfavorable standard free energy change HAD to spontaneously occur when the scientists orginally measured the standard free energy change that they recorded in the free energy tables that one might look up.
what is the difference bw standard free energy chance and free energy of a reaction?
the standard free energy change describes the energy change from Q1 to Keq at standard conditions. the free energy of a reaction tells us the change in energy from any Q, and it doesn't have to be at standard conditions
why are near equilibrium reactions easily reversible? why are some reactions considered irreversible? for example, ATP hydrolysis and aldolase have similar free energy changes, but aldolase is reversible.
near equilibrium reactions are easily reversible bc the free energy changes associated w/ them are relatively small. therefore, according to le chatlier's principle seemily unfavorable reactions can act favorably if Q is changed. some reactions are considered irreversible bc the -dG^0 is so large that the activation energy required for the reverse reaction to reach the transition state. in other words, there are so few product molecules, if any, which are able to achieve a high enough state to reach the transition state in the reverse direction.
----an irreversible reaction is a reaction that never reaches equilibrium. there are two reasons that this might happen, and often both are at play. the first and most common is the magnitude of the free energy change. draw a free energy diagram for the reaction A<-> B. if the dG^0 is greater than -30 kJ/mol, then the reverse rate has a huge energy hill to climb for the reverse energy (-30 kJ/mol plus the activation energy). there are so few product molecules with enough energy to go back to the reactant, that it is insignificant. in other words, it is so very slow that it is irreversible with regard to time. the second reason is the solubility of the product. if the product is poorly soluble, then it will fall out of the solution before the concentration gets high enough to reach equilibrium. an additional, but related problem occurs in living cells, where either the product is consumed by other reactions, or the product is toxic at high concentrations.
what is the relationship bw the forward and reverse rates for a near equilbrium reaction? what is the difference bw a rate (or reaction velocity) and a rate constant?
for a reaction that is at equilibrium, the rate of product formation is equal to the rate of the reactant formation. rate constants are unique to each specific reaction (they are unchanging, hence why its a constant). reaction velocity describes how fast a reaction is happening. the closer you get to equilibrium, the slower the reaction rate (or the reaction velocity decreases)
impression of the protein core that was discussed in lecture.
- linear polypeptide folded into its native state when in water
- folded bc of covalent and noncovalent interactions of the side chains and water solvent to be stable conformation
-native state has empty spaces due to the atoms' spherical shape and exert VDW forces. The water molecules are too big to fit in
- incredibly dense
- the functional group: peptide bonds source of most of the hydrogen bonding because it can act as a donor and acceptor in hydrogen bonding
-hydrophilic and polar interaction is the surface while the core is hydrophobic and nonpolar interactions because of the side chains with water solvent
draw free energy plot for reaction w/ k<1, k>1, and k=1. are the reactions favorable? can k be negative?
k<1: reactants lower than products: unfavorable
k=1: reactants and products equal w/ small hill: equilibrium.
k>1: products lower than reactants: favorable
for a reaction @ equilibrium, how would you calculate the standard free energy change?
dG^0= -RT in Keq
------ standard free energy change
bc dG= dg^0 + RT ln Q is the free energy change of the reaction equation
what is the difference bw the reaction quotient Q and Keq?
they both are ratios of concentrations of products to reactants.
Q: is the concentrations at equilibrium
Keq: concentrations at any point during the reaction
The standard free energy change for ATP hydrolysis at 37° C is -30.5 kJ mol-1 (T=310.15 K, R= 8.314× 10-3kJ K-1 mol-1): ATP <-> ADP + Pi (For hydrolysis reactions we ignore the contribution of water in the math). A red blood cell has the following concentrations of ATP (2.25 mM), ADP (0.25 mM) and Pi (1.65 mM). What is Q for this reaction? What is the dG of the reaction under these conditions?
mM has to be converted to Moles in order for the concentrations to be used for Q. 1 mM = .001 mole
Q=[product]/[reactant]= [.00025][.00165]/[.00225] = 1.65E^-4
dG = dG^0 + RT lnQ = -30.5 + (310.15)(8.314E^3)(1.65E^-4)= -52.9 kjmol^1.
consider the 2-state model for protein folding. using the rule of thumb, what is the dGfolding in kcal mol^-1 for a protein that exists in a ratio of 1000:1 folded:unfolded states at room temp? if a mutation destabilized the protein by 1.36 kcal mol^1, what could be the new ration of folded:unfolded?
dGfolding for a protein for ratio 1000:1 would be ln 1000 which would be a 3. so 3 times the -1.36 kcalmol^-1 = -4.08 kcal/mol
destabilized protein would be decreases the ratio bw the products and reactants. therefore the ratio would become a fold smaller than it currently is. 1.36 kcalmol^-1 is the same as 5.7 kjmol^-1 therefore the decrease would be 1 tenfold. the new ratio is 100:1 products:reactants.
3 non-covalent interactions and rank them by their approximate strength. identify the interactions that involve formal charges, dipole-dipole and induced dipole.
3 noncovalent interactions: hydrogen bonding, VDW, and Salt bridges/ionic bonds
strongest: salt bridges> HB> VDW weakest
salt bridges= formal charges
hydrogen bonding= dipole-dipole interactions
VDW= induced dipole
what two non-covalent interactions does water make w/ proteins or unfolded peptides?
hydrogen bonding and salt bridges/ionic bonds
if you observe a nucleophilic substitution reaction: AB + C <-> AC + B. imagine you are holding a beajer in which this reaction is occurring. How could you easily tell which bonds were stronger AB or AC?
enthalphy (dH).
breaker is hot: exothermic rxn: the AC bonds are stronger than the AB
breaker is cold: endothermic rxn: AB bonds are stronger than the AC
why are Hydrogen bonds bw water molecules in ice stronger than those in the liquid state? is ice melting at 25^0 C driven by enthalpy or entropy or both? is it exothermic?
they are stronger in ice bc they are in a lattice pattern due to the water molecule having four hydrogen bonds---partial covalent bonds. w/ ice, the molecules are flickering bw H3O, H2O, OH states which is causing the lattice pattern. liquid water's HB isnt as strong bc those molecules can only be in roughly 3 HBs.
ice melting @ 25^0C is driven by entropy. its favorable and spontaneous.
the rxn is endothermic, not exothermic. the rxn absorbs heat.




